Meet Regulatory Requirements and Development Needs.
Metronome Sentry delivers AI-powered governance with real-time response: Track compliance across developer work streams with audit-ready documentation and real-time risk visibility.
HIPAA Compliant
Built for SAFe®
DOD ATO-C Approved
FedRAMP Ready

- The Problem
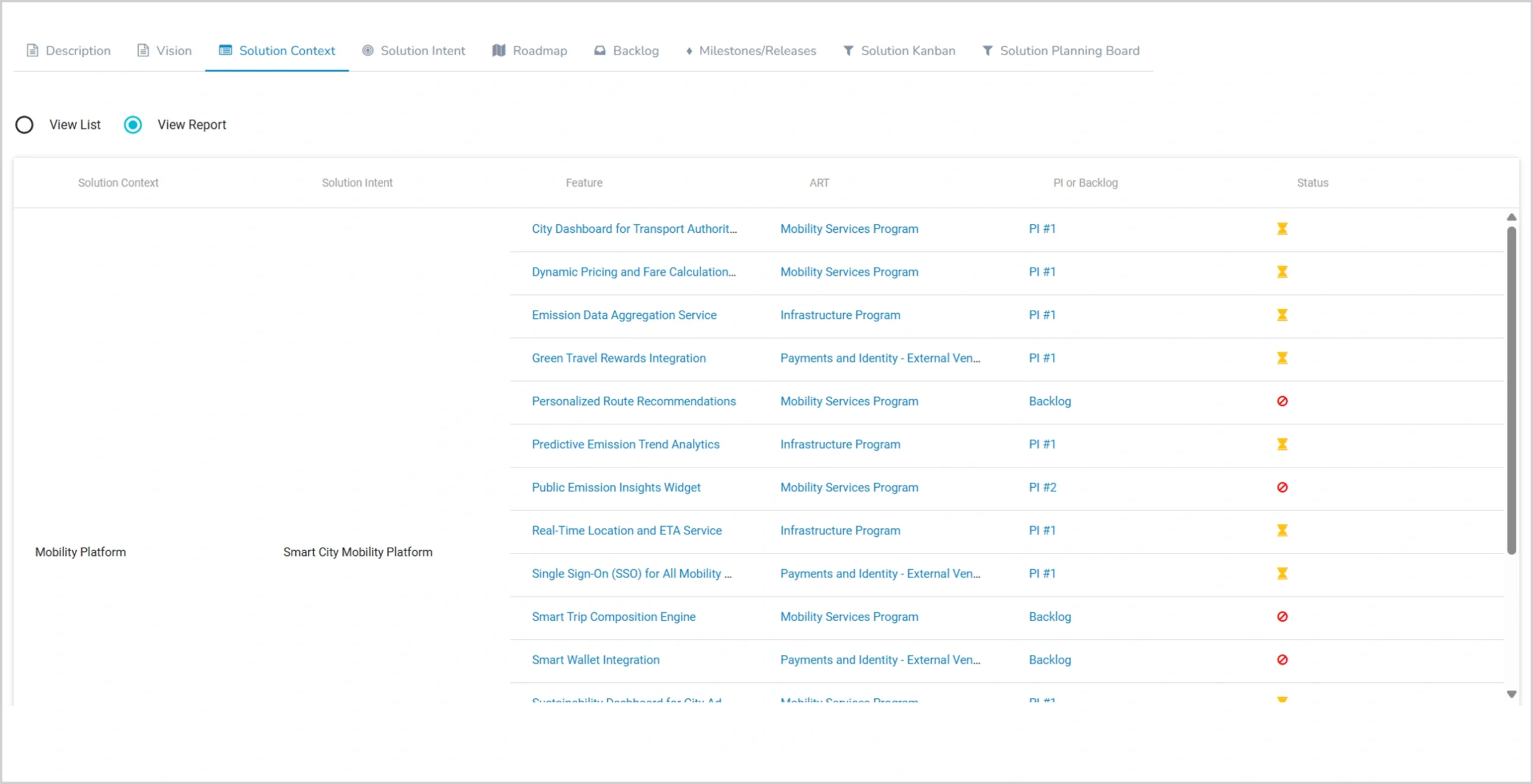
The Compliance Challenge
Auditors arrive next week. Your team is scrambling to prepare for their questions:
- Who identified risks and when?
- What impact analysis was performed?
- Who approved mitigation strategies?
- How was effectiveness measured?
Most Agile tools track features and sprints. Not the compliance trail you actually need.
- The Solution
Mitigate Compliance Risks in Highly Regulated Sectors
- Security & Compliance: PHI exposure, HIPAA violations, audit readiness gaps
- Clinical Integration: EHR dependencies, clinical approval delays, regulatory timelines
- Operational: Vendor dependencies, legacy system complexity, workflow disruption
Track compliance risks alongside delivery risks — both can halt your project.
- Predictive Alerts
Continuos Monitoring
Risk Management That Never Sleeps
- Real-time tracking of blockers, dependencies, and velocity
- Automated alerts when risk exceeds thresholds
- Daily risk score updates
- CI/CD integration for technical risks
Example alert:
Risk score for Epic 7.3 increased from ‘Medium’ to ‘High’ due to:
(1) Clinical approval dependency delayed 14 days,
(2) HIPAA security review not yet scheduled,
(3) Vendor integration blocked.
Audit trail attached.



- Workflow
Audit-Ready Documentation
Every risk decision is documented automatically. Here's what auditors see:
Sample Audit Record:
Risk ID: RISK-2025-0847
Identified by: J. Martinez (RTE) — 2025-11-03
Impact Analysis: Completed 2025-11-05
Mitigation approved by: S. Chen (PMO) — 2025-11-07
Status: Mitigated — 2025-11-19
Effectiveness review: Scheduled 2025-12-15

This is what auditors actually ask for. Metronome Sentry generates it automatically.
- Trust
Security & Compliance Certifications
HIPAA Compliant
Built for SAFe®
DOD ATO-C Approved
FedRAMP Ready
- HIPAA/HITRUST Compliant: Your team can work with PHI securely without workflow restrictions.
- DOD ATO-C Approved: Federal-grade security standards for government and regulated industries.
- Hybrid AI Security: Sensitive data processed locally. Intelligence delivered from the cloud. Best of both worlds.
- Cloud-Native Deployment: Deploy on AWS, Azure, or GovCloud with high availability and enterprise scalability.
- Project Management
Healthcare Product Planning
Built for Clinical and Technical Complexity
EHR Integration Planning
Native visibility into Epic, Cerner, and MEDITECH dependencies. Plan HL7/FHIR integrations with confidence.
PHI-Secure Planning
HIPAA/HITRUST compliant. Plan products that handle PHI without compliance concerns.
Regulatory Milestone Tracking
FDA submissions, security assessments, and compliance audits — track regulatory dependencies alongside feature delivery.
- Demo
See It In Action
Schedule a 30-minute demo and we’ll show you:
- Compliance tracking and audit trail generation
- Predictive risk alerts
- Regulatory milestone tracking
- Security and PHI management
Bring your toughest compliance requirement – we’ll show you how we track it.
AI-Powered
Built for Scrum
HIPAA Compliant
- FAQ
Frequently Asked Questions
Does Metronome Sentry integrate with existing risk registers?
Yes. Import data or sync bidirectionally with tools such as ServiceNow.
Does Metronome Sentry track non-technical risks?
Yes. Business, vendor, regulatory, and clinical approval risks are all tracked with full audit trails.
What about AI and PHI?
We have a hybrid architecture: local models for sensitive data, cloud models for intelligence. PHI stays secure and compliant.
- Next Steps
Stop Scrambling During Audits.
Join healthcare organizations with complete compliance visibility and audit-ready documentation built into their Agile workflow.